Contribution of constitutional liquation of the segregation phase to improve the electrochemical performance of Al–Sn based anodes†
Abstract
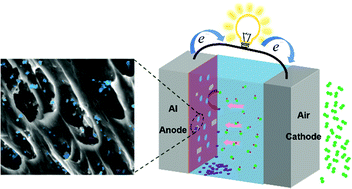
Based on the strategy of regulating the constitutional liquation of segregation phases, Al-based anode materials with a high voltage in the neutral electrolyte are designed and prepared for the Al–air battery. According to the Ga–In–Sn ternary phase diagram, the liquid segregation phase is designed and constructed inside the Al anode to improve the comprehensive performance of Al anodes. By adjusting the content of Ga and In, the obtained anodes with various liquid phase content in the grain boundaries present different electrochemical properties. The open-circuit potential of the optimal quaternary anode reaches −1.626 V in 2 M NaCl solution. A series of analyses, including phase composition, microstructure, element distribution and thermodynamic properties, are employed to successfully reveal the presence of the low-melting-point segregation phases and the influence on the electrochemical characteristics of these Al anode materials. It is found that the liquid segregation phase formed by alloying elements, Ga, In, and Sn, can effectively activate the Al anode and contribute to the discharge process. Furthermore, the activation mechanism of the Al anode driven by the liquid segregation phase is proposed.


 Please wait while we load your content...
Please wait while we load your content...