Bimetallic Ni–Mo nitride@N-doped C as highly active and stable bifunctional electrocatalysts for full water splitting†
Abstract
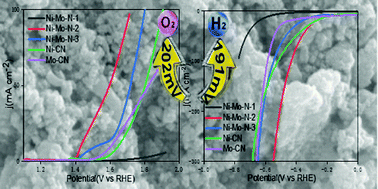
Designing low-cost, highly active and stable electrocatalysts is important to various renewable energy storage and conversion devices. We fabricated a NiMoN nanosheet hybrid catalyst through a reduction reaction of NiMoO4 nanosphere and urea under high-temperature annealing. The effects of annealing temperature on the surface chemical compositions of the catalyst were investigated, and it was found that the relative contents of various N-type species, Ni and Mo changed with the temperature. The relative contents of metal–N, metallic Ni, Mo3+ and Mo4+cations were increasing with the temperature increment, and significantly improved the activity of electrocatalytic water splitting. To drive a current density of 10 mA cm−2, the bimetallic NiMoN requires an overpotential of 202 mV and 191 mV for the OER and HER, respectively. The two-electrode system using the NiMoN needs a very low cell potential of 1.58 V to reach a current density of 10 mA cm−2. The catalyst also shows high durability in the case of 40 hours, while the overpotential is only slightly attenuated. The superior activity and long-term stability demonstrate that the NiMoN catalyst has promising potential for application in large-scale water splitting.


 Please wait while we load your content...
Please wait while we load your content...