Selective approach to N-substituted tertiary 2-pyridones†
Abstract
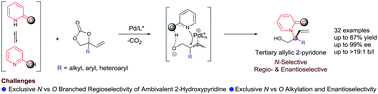
The regio- and enantioselective amination of readily available vinyl cyclic carbonates with ambivalent 2-hydroxypyridines has been achieved using a Pd(0)/DACH-naphthyl catalyst system. The reaction proceeds under simple conditions, providing access to chiral N-substituted 2-pyridones that bear elusive tertiary carbon centers. A significant drawback previously related to making these products via transition metal-catalyzed allylic substitution procedures is overcome by this catalytic method.


 Please wait while we load your content...
Please wait while we load your content...