Investigation of the thermodynamic properties of hydrates as cooling phase change materials for their implementation in electric vehicles
Abstract
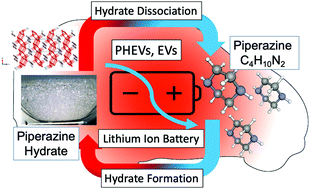
Electric vehicles (EVs) play key roles in realizing a sustainable society. Lithium-ion batteries (LIBs) implemented in EVs deteriorate due to significant temperature changes. To maintain LIBs in an appropriate temperature range, a competitive thermal energy storage (TES) medium is needed. Phase change materials (PCMs) that can utilize their latent heat have great potential. In this study, we focused on piperazine hexahydrate as a promising candidate for a PCM and investigated its thermodynamic properties that are essential for practical design. The equilibrium temperature and dissociation enthalpy were determined in the mass fraction range of 0.250 to 0.550. The results showed that piperazine hexahydrate had a maximum equilibrium temperature of 42.9 °C and a maximum dissociation enthalpy of 250.0 ± 3.7 kJ kg−1 at a mass fraction of 0.443, which is the congruent point of piperazine hexahydrate. Piperazine hexahydrate can be utilized as a PCM in the temperature range of 34.4 °C to 42.9 °C. By utilizing piperazine hexahydrate instead of a conventional water-cooling system, the volume of the TES tank may be reduced by 31% approximately. The crystal system of the piperazine hexahydrate prepared in the present study was determined to be pseudomonoclinic by powder X-ray diffraction (PXRD) measurements, which is the same as that reported in the literature.


 Please wait while we load your content...
Please wait while we load your content...