High-efficiency radon adsorption by nickel nanoparticles supported on activated carbon†
Abstract
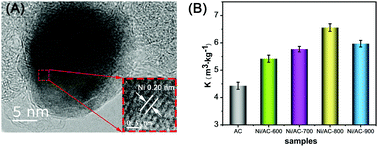
Radon (Rn) is a universally known indoor radioactive gas that poses a significant threat to human health. Activated carbon (AC) is the only commercial Rn adsorbent; its adsorption performance is extremely limited due to too few micropores. A series of nickel nanoparticles supported on AC (Ni/AC) composites, combining abundant micropores with open metal sites, is rationally designed for adsorbing Rn via a two-step process consisting of impregnation and high-temperature reduction. The Rn adsorption performance of the Ni/AC composites is obviously improved due to the introduction of nickel nanoparticles. The adsorption coefficient of Ni/AC calcined at 800 °C reaches 6.56 ± 0.14 m3 kg−1 at 25 °C and 1 bar, 48% higher than that of AC. The Ni/AC Rn adsorption coefficient is similar over five cycles. The fine and homogeneous dispersion of nickel nanoparticles, the appropriate chemical state of nickel and the high content of Ni0 synergistically promote Rn adsorption on Ni/AC. Experiments verify that Ni/AC composites are a promising candidate for the adsorption of Rn in the atmospheric environment.


 Please wait while we load your content...
Please wait while we load your content...