Room temperature ferroelectric copper(ii) coordination polymers based on amino acid hydrazide ligands†
Abstract
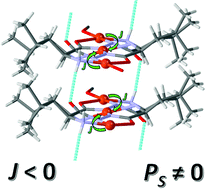
The ability of amino acid-based hydrazide ligands to produce polar crystalline materials that could possess technologically important physical properties, such as ferroelectricity, is tested. Two new coordination polymers {Cu3(L1/L2)2(TPA)}n×solvent were prepared in solvothermal reaction of copper(II) ions, terephthalic acid (TPA) and amino acid-based hydrazide ligands (H2L1 = N,N′-di-L-leucine hydrazide; H2L2 = N,N′-di-L-phenylalanine hydrazide). Structural analysis showed that both coordination polymers crystallize in an enantiomorphic polar point group 1 (C1) at room temperature. The assembled coordination polymers are neutral and consist of three copper(II) ions bridged with two amino acid-based hydrazide molecules, which in turn are linked with TPA molecules to form an overall one-dimensional structure. The magnetic order in these compounds is an antiferromagnetic interaction between copper(II) ions bridged by a hydrazide (–N–N–) bridge. Thermal decomposition of the two coordination polymers begins above 480 K, confirming the high thermal stability of the amino acid hydrazide ligands. The DSC shows that both compounds undergo a reversible phase transition around 388 K. The two coordination polymers show electrical conductivities of less than 10−10 S cm−1, with no change in the real part of the dielectric constant throughout the studied temperature range. Since both compounds crystallize in the ferroelectric space group, the occurrence of spontaneous polarization was tested by polarization–voltage hysteresis experiments.


 Please wait while we load your content...
Please wait while we load your content...