Urease inhibitory kinetics, molecular docking, SAR and ADME studies of imine analogues†
Abstract
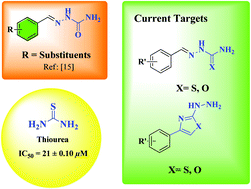
A series of synthesized imine derivatives (3a–m), including thio-semicarbazone, semicarbazone, thiazole and oxazole functional moieties, were examined for in vitro urease inhibition activity. Among all the analogues, 3b, 3k and 3f were the most potent against urease, exhibiting IC50 values of 1.52 ± 0.026, 2.20 ± 0.018 and 2.94 ± 0.058 μM, respectively. Docking studies were performed for the potent inhibitors to demonstrate their binding interactions with urease. Kinetic studies were also performed to assess the mode of interaction of the most potent analogue with urease. Moreover, an in silico ADME study was performed to evaluate the drug likeness of the compounds. All the analogues showed favorable ADME profiles.


 Please wait while we load your content...
Please wait while we load your content...