Integrating a covalent probe with ubiquicidin fragment enables effective bacterial infection imaging†
Abstract
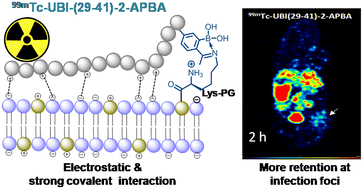
Developing potent and novel bacterial imaging agents remains formidable due to the rapid development of bacterial resistance. Ubiquicidin and its derivatives are the most studied antimicrobial peptides that bind to anionic membranes of a broad range of bacterial pathogens. Studies reveal that UBI (29-41) labeled with 99mTc and 68Ga could distinguish sterile inflammation from infection. A significant challenge that remains for cationic peptides is their poor salt tolerance. The present study deliberates the increment of UBI (29-41) peptide interaction with the bacterial membrane by incorporating 2-acetylphenylboronic acid (2-APBA) as a covalent probe and developing infection imaging probes with improved retention at the target. Given that both 99mTc-UBI (29-41) and 99mTc-UBI (29-41)-2-APBA peptide complexes are stable in serum over 16 h, 99mTc-UBI (29-41)-2-APBA shows enhanced uptake in S. aureus cells as compared to 99mTc-UBI (29-41). SPECT imaging in a mouse model of infection exhibited a higher target to non-target ratio after 2 h in the case of 99mTc-UBI (29-41)-2-APBA. The present study reveals a synergistic mechanism of target binding through covalent conjugation and non-covalent interaction, which could be a potential strategy for improving bacterial infection imaging. As a proof of concept, 99mTc-UBI (29-41)-2-APBA elicits our hypothesis by in vivo imaging of bacterial infection.
- This article is part of the themed collection: Covalent Drug Discovery


 Please wait while we load your content...
Please wait while we load your content...