Structure-based discovery of potent inhibitors of Axl: design, synthesis, and biological evaluation†
Abstract
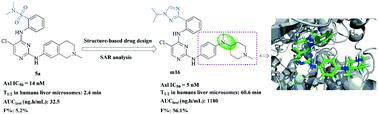
Commonly overexpressed in many cancers and associated with tumor growth, metastasis, drug resistance, and poor overall survival, Axl has emerged as a promising target for cancer therapy. However, the availability of new chemical forms for Axl inhibition is limited. Herein, we present the development and characterization of novel Axl inhibitors, including the design, synthesis, and structure–activity relationships (SARs) of a series of diphenylpyrimidine–diamine derivatives. Most of these compounds exhibited remarkable activity against the Axl kinase. In particular, the promising compound m16 showed the highest enzymatic inhibitory potency (IC50 = 5 nM) and blocked multiple tumor cells' proliferation potencies (the CC50 of 4 out of 42 cancer cell lines <100 nM). Furthermore, compound m16 also possessed preferable pharmacokinetic profiles and liver microsome stability. All these favorable results make m16 a good leading therapeutic candidate for further development.


 Please wait while we load your content...
Please wait while we load your content...