Depolymerization of lignin for biological conversion through sulfonation and a chelator-mediated Fenton reaction†
Abstract
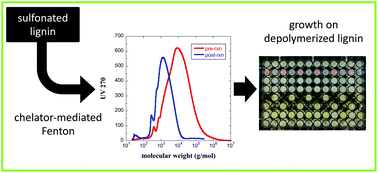
Generating value from lignin through depolymerization and biological conversion to valuable fuels, chemicals, or intermediates has great promise but is limited by several factors including lack of cost-effective depolymerization methods, toxicity within the breakdown products, and low bioconversion of the breakdown products. High yield depolymerization of natural lignins requires cleaving carbon–carbon bonds in addition to ether bonds. To address that need, we report that a chelator-mediated Fenton reaction can efficiently cleave C–C bonds in sulfonated polymers at or near room temperature, and that unwanted repolymerization can be minimized through optimizing reaction conditions. This method was used to depolymerize lignosulfonate from Mw = 28 000 g mol−1 to Mw = 800 g mol−1. The breakdown products were characterized by SEC, FTIR and NMR and evaluated for bioavailability. The breakdown products are rich in acid, aldehyde, and alcohol functionalities but are largely devoid of aromatics and aliphatic dienes. A panel of nine organisms were tested for the ability to grow on the breakdown products. Growth at a low level was observed for several monocultures on the depolymerized lignosulfonate in the absence of glucose. Much stronger growth was observed in the presence of 0.2% glucose and for one organism we demonstrate doubling of melanin production in the presence of depolymerized lignosulfonate. The results suggest that this chelator-mediated Fenton method is a promising new approach for biological conversion of lignin into higher value chemicals or intermediates.


 Please wait while we load your content...
Please wait while we load your content...