Chemo- and regio-selective amidation of indoles with isocyanates using borane Lewis acids†
Abstract
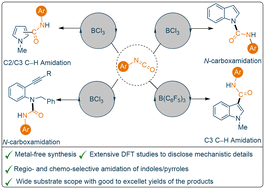
The efficacy of boron-based catalysts has drawn considerable attention from the scientific community due to their relatively low toxicities and high selectivities. Formation of a new carbon–carbon or carbon–nitrogen bond to generate an amide/urea functionality using mild, catalytic reaction protocols has always been an important challenge, as functionalised amides and urea derivatives are important scaffolds in medicinal chemistry. Herein we report a facile and mild catalytic reaction protocol towards the amidation of N-methyl indoles/pyrroles (17 examples, yields up to 58%) using B(C6F5)3 (30 mol%). Moreover, our investigation revealed that although catalytic amounts of B(C6F5)3 (10 mol%) are efficient towards the N-carboxamidation of unprotected indoles, catalytic BCl3 (5 mol%) is capable of producing near quantitative yields of the N-carboxamidation products (21 examples, yields up to 95%). In contrast with previous literature reports, the reaction between 2-(alkynyl)anilines and aryl isocyanates using catalytic BCl3 (5 mol%) afforded N–H inserted products (9 examples, yields up to 71%) chemo-selectively as opposed to the intramolecular hydroamination product. Comprehensive DFT studies have been undertaken to understand the mechanistic details of the N–H functionalisation of indoles.
- This article is part of the themed collection: Celebrating our 2025 Prizewinners


 Please wait while we load your content...
Please wait while we load your content...