Adjusting the match-degree between electron library and surface-active sites and forming surface polarization in MOF-based photo-cocatalysts for accelerating electron transfer†
Abstract
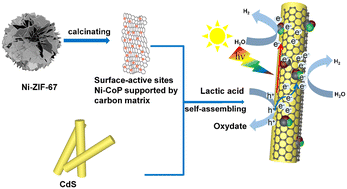
For the purpose of revealing the mechanism of photogenerated electron transfer in a modified metal–organic framework (MOF) based cocatalyst in photocatalytic hydrogen production (PHP), Ni-CoP supported by a carbon matrix as the cocatalyst is synthesized and loaded onto the CdS host catalyst by means of self-assembly. The degree of matching between the carbon matrix as the electron library and nanoscale clusters of Ni-CoP as surface-active sites is thoroughly investigated, which is triggered by temperature-tuning and heteroatom substitution respectively. The catalytic mechanism could be translated as that, when the photogenerated electrons are excited, internally driven by surface polarization due to the redistribution of charge density around the Fermi level of Ni-CoP, photogenerated electrons are transferred from the host catalyst to the electron library of the cocatalyst for storing and are available for use by the surface-active sites. Importantly, the synergistic effect of the highly matched-degree between the electron library and surface-active sites improves the electron utilization efficiency, and consequently contributes to better performance of PHP. The text shows that the PHP of 7% Ni-CoP/CdS is 28 357 μmol h−1 g−1, which is 2.2 times that of 7% CoP/CdS (13 078 μmol h−1 g−1) and 15.1 times that of pure CdS (1873 μmol h−1 g−1). This will be a promising approach to design efficient cocatalysts for achieving solar-to-chemical energy conversion.


 Please wait while we load your content...
Please wait while we load your content...