Cerium versus zirconium UiO66 metal–organic frameworks coupled with CdS for H2 evolution under visible light†
Abstract
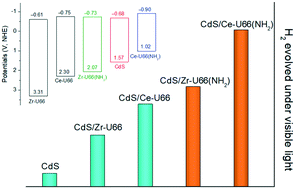
Metal–organic frameworks (MOFs) as highly porous photocatalysts have received increasing attention. Among them, much work has focused on UiO66, due to its high stability in water. In this work, four UiO66-based MOFs have been compared as photocatalysts for H2 production in an aqueous suspension of Na2S/Na2SO3. Under a 420 nm LED lamp, the amount of H2 evolved at 2 h (n2h) was negligible for all MOFs. After 40% cubic CdS loading via a two-step method, the n2h notably increased, which varied in the order Ce-UiO66-NH2 > Zr-UiO66-NH2 > Ce-UiO66 > Zr-UiO66. By contrast, CdS and 0.5% Pt/Ce-UiO66-NH2 had an n2h value approximately 12 and 17 times smaller than that measured for 40% CdS/Ce-UiO66-NH2, respectively. Among the MOFs, Ce-UiO66-NH2 had the smallest band gap, the most negative flat band potential, and the fastest charge transfer at a solid–liquid interface. A possible mechanism is proposed, involving the mutually promoted charge transfer between Ce-UiO66-NH2 and CdS, followed by proton reduction on CdS and sulfide oxidation on the MOF. This work shows that Ce-based UiO66 is more active than a Zr-based one coupled with CdS for H2 production under visible light.


 Please wait while we load your content...
Please wait while we load your content...