In situ reconfiguration of plasma-engineered copper electrodes towards efficient electrocatalytic hydrogenation†
Abstract
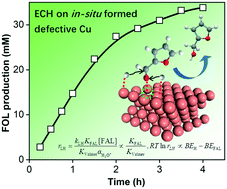
As a new route for biomass upgrading, electrocatalytic hydrogenation (ECH) requires a catalyst surface/subsurface that optimizes intermediate chemisorption towards target pathways. Defect engineering of Cu electrodes via plasma treatment is herein introduced to accomplish the efficient ECH of furfural (FAL) to furfuryl alcohol (FOL), thanks to the boosted kinetics on in situ reconfigured and defective Cu. Our experimental and theoretical analysis points out that the rate of ECH is positively correlated with the difference between the binding energies of chemisorbed H and FAL regarding their competitive adsorption, and thereby the higher discrepancy on defective Cu enables fast ECH in comparison with defect-free Cu. O2-plasma treatment on Cu foil (OP-Cu) produces CuOx that quickly undergoes in situ reduction to Cu with high roughness and rich defects during the initial ECH. Thereby, OP-Cu affords a high Faradaic efficiency (91%) of FOL at −0.56 V vs. RHE, superior to that of electropolished or Ar-plasma treated Cu. Its high efficacy identified in a wide substrate scope further enlightens catalyst design for electrochemical refinery via dynamic control.


 Please wait while we load your content...
Please wait while we load your content...