Oxygen nonstoichiometry and thermodynamic quantities of Gd and Cu doped misfit-layered calcium cobaltites
Abstract
Hyperstoichiometric (p-type) misfit-layered calcium cobaltites have been studied as components in various high-temperature electrochemical devices. Multiple studies have reported their applications or physical properties, but systematic studies on their defect structures and thermodynamic quantities are still insufficient. In this study, the oxygen nonstoichiometry and the electrical conductivity of Gd–Cu co-doped misfit cobalt oxide were measured as functions of temperature and oxygen partial pressure, along with thermodynamic quantities. The behavior of oxygen nonstoichiometry could not be explained by a defect structure assuming the ideal solution, as it showed a positive deviation in Raoult's law. The redesigned nonideal proposed defect structure, considering that the deviation originated from the high concentration of degenerate holes, could describe the oxygen nonstoichiometry precisely; and in this process, the values of 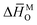 ,
, 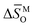 , Nv,
, Nv, 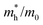 , γh, and
, γh, and 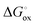 were quantitatively extracted. These values were compared with those obtained for the undoped system. The total electrical conductivity was measured using a dense specimen obtained via spark plasma sintering, and the anisotropic nature of the material was confirmed.
were quantitatively extracted. These values were compared with those obtained for the undoped system. The total electrical conductivity was measured using a dense specimen obtained via spark plasma sintering, and the anisotropic nature of the material was confirmed.



 Please wait while we load your content...
Please wait while we load your content...