Carbon-based frustrated Lewis pairs mediate hydrogenation†
Abstract
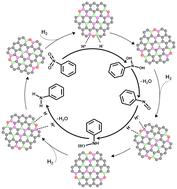
Hydrogen activation and the consequent catalytic hydrogenation of nitrobenzene on metal-free catalysts have been an attractive alternative to traditional metal-based catalytic process. Here we design a type of B/P co-doped nanocarbon (BPC) catalyst via the engineering of frustrated Lewis pairs (FLPs) on nanocarbons. It exhibits excellent catalytic activity with 97% conversion and 98% selectivity in hydrogen assisted hydrogenation of nitrobenzene to form aniline, which is superior to that of pure carbon and single B or P doped carbon materials. Structural characterizations, kinetic measurements and density functional theory (DFT) results indicate that Lewis acid (“B” center) and Lewis base (“P” center) engineered FLP sites on the BPC are responsible for the formation of aniline, and the moderate P/B ratio is important in promoting the hydrogenation activity. Moreover, we put forward a possible reaction mechanism and point out the key factors in determining the reactivity for this reaction. The current work provides a facile strategy for developing highly efficient hydrogenation catalysts.


 Please wait while we load your content...
Please wait while we load your content...