Ultrafast study of substituted-position-dependent excited-state evolution in benzophenone-carbazole dyads†
Abstract
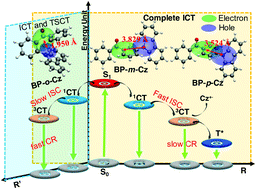
Donor and acceptor (D–A) compounds based on benzophenone (BP) and carbazole (Cz) were recently reported to exhibit an extraordinary long afterglow phosphorescence in the solid state. However, the BP derivatives’ mechanism of long afterglow phosphorescence is obscure. BP-o-Cz, BP-m-Cz, and BP-p-Cz were designed by coupling Cz at the ortho-, meta- and para-positions of the BP's benzene ring to uncover the excited-state evolution of BP-Cz molecules. Femtosecond and nanosecond transient absorption and excited-state theoretical calculations were carried out to detect and trace the photophysical process of BP-Cz dyads. After the excitation, all dyads experience intramolecular charge transfer (ICT) and intersystem crossing (ISC) processes. The resulting charge-transfer (1CT and 3CT) state of BP-o-Cz will decay to the ground state directly and quickly via the fast charge recombination (CR) process, which may be caused by through-space D–A interaction due to the enforced proximity between BP and Cz. In contrast, for BP-m-Cz and BP-p-Cz dyads, the complete separation of HOMOs and LUMOs leads to extended ICT and slow CR processes, producing an obvious Cz cation radical intermediate and an ultralong-lived triplet state species after the 3CT. Herein, we demonstrated that the excited-state evolution channels could be modified by tuning the substituted positions of D–A dyads. This may pave the way for designing efficient D–A type luminescent materials.


 Please wait while we load your content...
Please wait while we load your content...