Reverse water–gas shift reaction catalyzed by diatomic rhodium anions†
Abstract
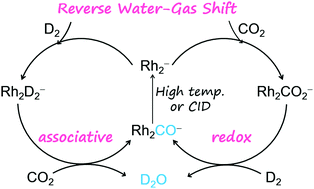
The reverse water–gas shift (RWGS, CO2 + H2 → CO + H2O, ΔH298 = +0.44 eV) reaction mediated by the diatomic anion Rh2− was successfully constructed. The generation of a gas-phase H2O molecule and ion product [Rh2(CO)ads]− was identified unambiguously at room temperature and the only elementary step that requires extra energy to complete the catalysis is the desorption of CO from [Rh2(CO)ads]−. This experimentally identified Rh2− anion represents the first gas-phase species that can drive the RWGS reaction because it is challenging to design effective routes to yield H2O from CO2 and H2. The reactions were performed by using our newly developed double ion trap reactors and characterized by mass spectrometry, photoelectron spectroscopy, and high-level quantum-chemical calculations. We found that the order that the reactants (CO2 or D2) were fed into the reactor did not have a pronounced impact on the reactivity and the final product distribution (D2O and Rh2CO−). The atomically precise insights into the key steps to guide the reaction toward the RWGS direction were provided.


 Please wait while we load your content...
Please wait while we load your content...