Why does 2-(2-aminoethylamino)ethanol have superior CO2 separation performance to monoethanolamine? A computational study†
Abstract
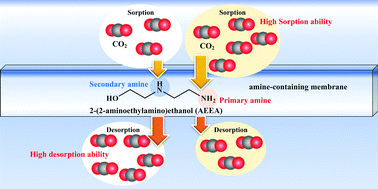
Our computational reaction analysis shows that 2-(2-aminoethylamino)ethanol (AEEA) has superior performance to monoethanolamine for CO2 separation, in terms of its ability to sorb CO2 by its primary amine and desorb CO2 by its secondary amine.


 Please wait while we load your content...
Please wait while we load your content...