Generation of singlet oxygen catalyzed by the room-temperature-stable anthraquinone anion radical†
Abstract
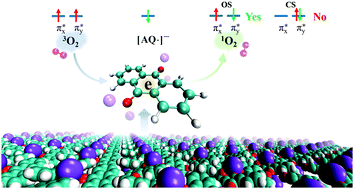
The chemical nature and the catalytic selectivity of the complex of anthraquinone and potassium tert-butoxide, AQ–KOtBu, in generating singlet oxygen (1O2) have been studied using a high-level ab initio method and density functional theory (DFT). The results suggest that the stable catalytic center of the AQ anion radical (semiquinone, [AQ˙]−) can be produced at room temperature, which is due to the strong delocalization characteristics of electrons in potassium atoms. Two experimentally observed complexes, the ground state AQ–KOtBu, i.e., C(1), and the photoexcited AQ–KOtBu, i.e., C(2), can be distinguished via the two different electronic states (π-type and σ-type) of the tert-butoxide group. More interestingly, the catalytic selectivity of AQ–KOtBu to generate 1O2 was investigated using multistate density functional theory (MSDFT), and the results suggest that only open-shell 1O2 rather than the closed-shell component can be generated. This work explores the electronic structure and the catalytic nature of AQ–KOtBu, which is of great importance for the application of AQ and its derivatives.


 Please wait while we load your content...
Please wait while we load your content...