Catalyst-free CO2 hydrogenation with BH3NH3 in water: DFT mechanistic insights†
Abstract
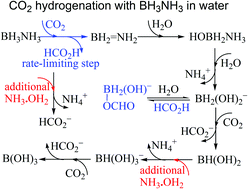
Extensive DFT calculations show that BH3NH3 may transfer dihydrogen as separated hydride and proton to CO2 rather than HCO3− in water over a barrier of 25.9 kcal mol−1, followed by faster hydride transfer from borate anions to either electrophilic CO2 or protic H2O or HCO2H, leading to competitive formate production and H2 release.


 Please wait while we load your content...
Please wait while we load your content...