Photoswitching activation of a ferrocenyl-stilbene analogue by its covalent grafting to gold†
Abstract
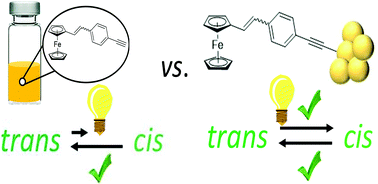
Until now, surface-deposited stilbenes have been much less studied than other photochromic systems. Here, an asymmetrically substituted styrene incorporating a redox-active ferrocene moiety and a terminal alkyne group has been synthesised to investigate its photoisomerization in solution, and upon the formation of chemisorbed self-assembled monolayers through a carbon–gold bond formation. Charge transport measurements across the monolayers reveal that upon chemical linkage to the gold substrate there is an alteration of the isomerization pathway, which favours the trans to cis conversion, which is not observed in solution. The experimental observations are interpreted based on quantum chemistry calculations.


 Please wait while we load your content...
Please wait while we load your content...