Critical size effect for the surface heat capacities of nano-CdS: theoretical and experimental studies†
Abstract
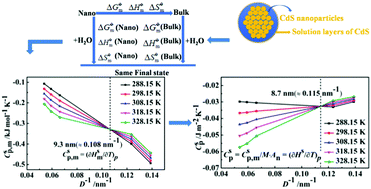
The unique physical and chemical properties of nanomaterials are closely related to their surface thermodynamic functions, which mainly depend on their sizes. In this study, the thermodynamic properties of nano-cadmium sulphide (nano-CdS) were investigated by solubility technology. The nano-CdS powders with different particle sizes were prepared via a traditional solvothermal method, and the electrical conductivities of nano-CdS aqueous solutions at different temperatures were measured. The standard dissolution equilibrium constants of nano-CdS at different temperatures were calculated using the theory of dissolution thermodynamics. The standard molar dissolution thermodynamic functions, the molar surface thermodynamic functions and the specific surface thermodynamic functions of nano-CdS with different particle sizes were calculated by combining the thermodynamic functions of bulk-CdS, the principle of the thermodynamic cycle and the principle of electrochemical equilibrium. The experimental results show that the critical size values for the molar surface heat capacity and the specific surface heat capacity for approximately spherical nanoparticles are 9.3 nm and 8.7 nm, respectively. Within an acceptable range of error, the thermodynamic functions have linear and curved relationships with particle sizes and temperatures. Based on these results, it is disclosed that the critical size effect on surface heat capacities of nano-CdS is valuable to understand the energy storage processes of nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...