Single-phase CZTSe via isothermal recrystallization in a KI–KCl flux
Abstract
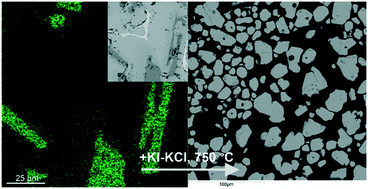
A facile, two-step procedure was developed to prepare a pure CZTSe (Cu2ZnSnSe4) phase from elemental Cu, Zn, Sn, and Se with the addition of a KI–KCl mixture. During the first step, the mixture charge was reacted at 1000 °C to convert all the metals to selenides. Next, the extracted charge was powdered and again heated to 750 °C to obtain homogeneous grains of CZTSe with a size of 30–100 microns. The main intermediate phase during the synthesis of Cu2SnSe3 was found to dissolve Zn up to 11.5 at% and, without Raman spectroscopy data, this phase could be easily confused with CZTSe because they are not optically distinguishable. On the other hand, the deviation of the Zn content in CZTSe does not exceed ±0.5 at%.


 Please wait while we load your content...
Please wait while we load your content...