Isothiourea-catalysed enantioselective radical conjugate addition under batch and flow conditions†
Abstract
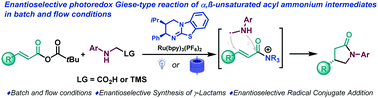
The photocatalytic generation of α-amino radicals is combined with chiral isothiourea derived α,β-unsaturated acyl ammonium intermediates. The reaction proceeds VIA a [3+2] radical-polar crossover mechanism to generate γ-lactams in good yields and enantioselectivities. The enantioselective radical conjugate addition was carried out under batch and flow conditions.


 Please wait while we load your content...
Please wait while we load your content...