Enantioselective synthesis of acyclic monohydrosilanes by steric hindrance assisted C–H silylation†
Abstract
Herein, a rhodium-catalyzed desymmetrization of dihydrosilanes with heterocyclic compounds via intermolecular dehydrogenative C–H silylation is developed. The strategy tolerates a variety of thianaphthene and thiophene derivatives, giving rise to a wide range of silicon-stereogenic acyclic monohydrosilanes. Several rare skeletons featuring bis-silicon-stereogenic centers were also designed to enhance the library's diversity further. Preliminary mechanistic studies reveal that the surrounding spatial environment of the Si-center plays a crucial role in enabling intermolecular C–H silylation preferentially.
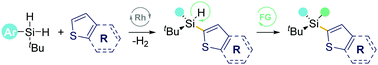


 Please wait while we load your content...
Please wait while we load your content...