A highly enantioselective approach towards optically active γ-amino alcohols by tin-catalyzed kinetic resolution of 1,3-amino alcohols†
Abstract
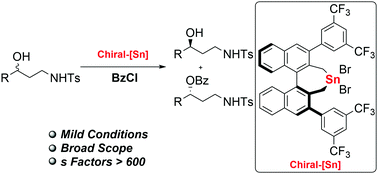
A highly enantioselective kinetic resolution of racemic 1,3-amino alcohols via O-Acylation was achieved using a chiral organotin as the catalyst. Alkyl- and aryl-substituted 1,3-amino alcohols were resolved with excellent efficiencies to afford the recovered 1,3-amino alcohols and acylative products with high enantioselectivities, with s factors up to >600. Notably, the chiral organotin catalyst was more selective for anti-1,3-amino alcohols than for syn-isomers. A Gram-scale reaction with loading using 2 mol% catalysts demonstrated the utility of this protocol.


 Please wait while we load your content...
Please wait while we load your content...