Crystalline radical cations of bis-BN-based analogues of Thiele's hydrocarbon†
Abstract
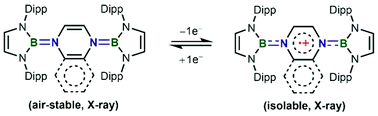
Two radical cations of bis-BN-based analogues of Thiele's hydrocarbons were facilely synthesized, fully characterized, and theoretically investigated. One-electron oxidation leads to the reduced bond length alternation and NICS values of the central C4N2 rings, suggesting the decreasing antiaromatic character. The spin density of the radical cations is significantly delocalized over the central linkers with a small contribution from two terminal N-heterocyclic boryl units.
- This article is part of the themed collection: ChemComm Milestones – First Independent Articles


 Please wait while we load your content...
Please wait while we load your content...