Stereospecific reaction of sulfonimidoyl fluorides with Grignard reagents for the synthesis of enantioenriched sulfoximines†
Abstract
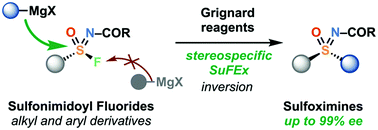
Sulfonimidoyl halides have previously shown poor stability and selectivity in reaction with organometallic reagents. Here we report the preparation of enantioenriched sulfonimidoyl fluorides and their stereospecific reaction at sulfur with Grignard reagents. Notably the first enantioenriched alkyl sulfonimidoyl fluorides are prepared, including methyl. The nature of the N-group is important to the success of the stereocontrolled sequence to sulfoximines.
- This article is part of the themed collection: 2022 Pioneering Investigators


 Please wait while we load your content...
Please wait while we load your content...