Copper-catalyzed asymmetric 1,6-conjugate addition of in situ generated para-quinone methides with β-ketoesters†
Abstract
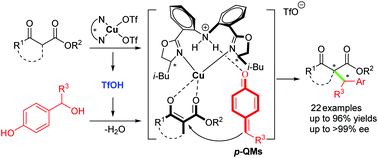
A Cu-catalyzed asymmetric 1,6-conjugate addition of in situ generated para-quinone methides (p-QMs) with β-ketoester has been developed to construct a ketoester skeleton bearing an adjacent tertiary–quaternary carbon stereocenter in good yields and high enantioselectivities. This is the first example of metal-catalyzed asymmetric transformations of the in situ generated p-QMs, avoiding using pre-synthesized p-QMs requiring bulky 2,6-substitutions and highlighting a new dual catalytic activation with the chiral bis(oxazoline)–metal complex acting as a normal Lewis acid to activate the β-ketoesters and a source of Brønsted acid responsible for generating the p-QMs in situ.


 Please wait while we load your content...
Please wait while we load your content...