Synthesis of chiral polycyclic N-heterocycles via gold(i)-catalyzed 1,6-enyne cyclization/intramolecular nucleophilic addition†
Abstract
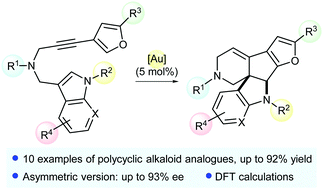
Cycloisomerization of N-tethered indole- and dihydropyrrole-arylpropargyl substrates into complex polycycles was investigated by using gold(I) catalysis. Enantioselectivities up to 93% ee were obtained in the asymmetric version of this process. DFT calculations rationalized the reactivity observed for the formation of such complex molecular frameworks.


 Please wait while we load your content...
Please wait while we load your content...