Catalytic hydroaminations of alkynes: a facile protocol to vinyl-carbazole derivatives via a frustrated Lewis pair mechanism†
Abstract
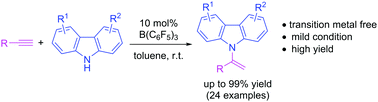
N-Vinylcarbazoles are important skeletons for photoluminescent materials. Herein, a transition metal-free, B(C6F5)3 mediated carbazolation reaction of alkynes is reported, providing 24 variants of N-vinylcarbazole derivatives. These N-vinylcarbazole products were obtained in good to excellent yields (up to 99%) under mild reaction conditions and could be performed on a gram scale.


 Please wait while we load your content...
Please wait while we load your content...