Amino acid sequence determines the adjuvant potency of a d-tetra-peptide hydrogel†
Abstract
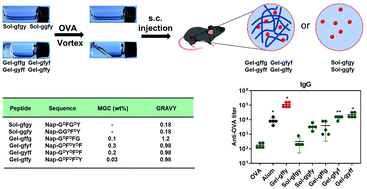
The development of novel vaccine adjuvants is essential for the production of modern vaccines against infectious agents and cancer. We recently reported a supramolecular hydrogel of a self-assembling D-tetra-peptide named Nap-GDFDFDY (Gel-gffy) that can evoke potent humoral and cellular immune responses; however, the determinants of its immunostimulatory properties were not characterized. In this study, we show that the amino acid sequence of the peptide determines the adjuvant potency of Gel-gffy. We designed and synthesized five Gel-gffy variants (Sol-gfgy, Sol-ggfy, Gel-gffg, Gel-gfyf, and Gel-gyff) by substituting the phenylalanine and tyrosine to glycine or changing the position of the tyrosine in the parent D-tetra-peptide. First, we characterized their gelation properties, nanomorphology, and secondary structure using transmission electron microscopy and circular dichroism; next, we examined their immunostimulatory properties. Gel-gfyf, Gel-gyff and Gel-gffy markedly upregulated maturation marker expression on bone marrow-derived dendritic cells. Moreover, the Gel-gfyf-, Gel-gyff- or Gel-gffy-encapsulated ovalbumin (OVA) vaccine induced robust humoral and cellular immune response in vivo. Notably, Gel-gffy had the strongest immunostimulatory activity. Our findings demonstrate that both the position and number of aromatic amino acids are crucial in determining the adjuvant potency of Gel-gffy, thus providing a valuable insight into designing peptide hydrogels as vaccine adjuvants.


 Please wait while we load your content...
Please wait while we load your content...