Determination of specific and non-specific protein–protein interactions for beta-lactoglobulin by analytical ultracentrifugation and membrane osmometry experiments†
Abstract
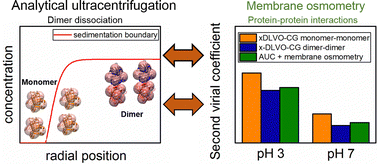
Protein–protein interactions are essential for the understanding of biological processes. Specific protein aggregation is an important aspect for many biological systems. In particular, electrostatic interactions play the key role for protein–protein interactions, as many amino acids have pH-dependent charge states. Moreover, protein dissociation is directly related to the solution pH, ionic strength, temperature and protein concentration. The subtle interplay between different specific and non-specific interactions is demonstrated for beta-lactoglobulin (BLG) with a focus on low salt concentrations, thus mimicking technically relevant processing conditions. BLG is a well-characterized model system, proven to attain its monomer–dimer equilibrium strongly dependent upon the pH of the solution. In this manuscript, we present a unique combination of analytical ultracentrifugation and membrane osmometry experiments, which quantifies specific and non-specific interactions, i.e. in terms of the dimer dissociation constants and the second osmotic virial coefficient, at pH 3 and 7 and sodium chloride concentrations of 10 mM and 100 mM. This provides direct insight to protein–protein interactions for a system with a concentration-dependent monomer–dimer equilibrium. Moreover, using a coarse-grained extended DLVO model in combination with molecular dynamics simulations, we quantify non-specific monomer–monomer, monomer–dimer and dimer–dimer interactions as well as the binding free energy of BLG dimerization from theoretical calculations. The experimentally determined interactions are shown to be mainly governed by electrostatic interactions and further agree with free energy calculations. Our experimental protocol aims to determine non-specific and specific interactions for a dynamically interacting system and provides an understanding of protein–protein interactions for BLG at low salt concentrations.


 Please wait while we load your content...
Please wait while we load your content...