Diastereoconvergent synthesis of anti-1,2-amino alcohols with N-containing quaternary stereocenters via selenium-catalyzed intermolecular C–H amination†
Abstract
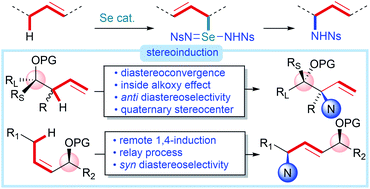
We report a diastereoconvergent synthesis of anti-1,2-amino alcohols bearing N-containing quaternary stereocenters using an intermolecular direct C–H amination of homoallylic alcohol derivatives catalyzed by a phosphine selenide. Destruction of the allylic stereocenter during the selenium-catalyzed process allows selective formation of a single diastereomer of the product starting from any diastereomeric mixture of the starting homoallylic alcohol derivatives, eliminating the need for the often-challenging diastereoselective preparation of starting materials. Mechanistic studies show that the diastereoselectivity is controlled by a stereoelectronic effect (inside alkoxy effect) on the transition state of the final [2,3]-sigmatropic rearrangement, leading to the observed anti selectivity. The power of this protocol is further demonstrated on an extension to the synthesis of syn-1,4-amino alcohols from allylic alcohol derivatives, constituting a rare example of 1,4-stereoinduction.


 Please wait while we load your content...
Please wait while we load your content...