Stepwise reduction of a base-stabilised ferrocenyl aluminium(iii) dihalide for the synthesis of structurally-diverse dialane species†
Abstract
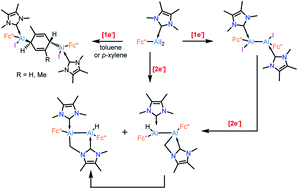
We report the reduction of bulky ferrocenyl-based NHC-stabilised aluminium(III) diiodide [Fc*(NHC)AlI2] (Fc* = 2,5-bis(3,5-di-tert-butylphenyl)-1-ferrocenyl) in different hydrocarbon solvents (hexane, benzene, toluene, and p-xylene), which results in different outcomes. Reduction in hexane with an equivalent amount of KC8 generates the diiododialane [(Fc*(NHC)AlI)2], whereas complete reduction in hexane leads to an unusual C–H activation at an N–Me group of one NHC unit. In contrast, reaction in aromatic solvents result in hitherto unknown Birch-type reductions of the corresponding solvent molecules by transient aluminium radicals of the type [LAlR2]˙, which is ultimately bound to two aluminium centers.


 Please wait while we load your content...
Please wait while we load your content...