Nickel-catalysed diversification of phosphine ligands by formal substitution at phosphorus†
Abstract
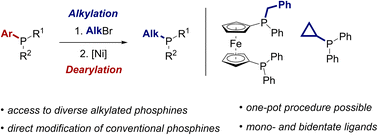
We report a diversification strategy that enables the direct substituent exchange of tertiary phosphines. Alkylated phosphonium salts, prepared by standard alkylation of phosphines, are selectively dearylated in a nickel-catalysed process to access alkylphosphine products via a formal substitution at the phosphorus center. The reaction can be used to introduce a wide range of alkyl substituents into both mono- and bisphosphines. We also show that the alkylation and dearylation steps can be conducted in a one-pot sequence, enabling accelerated access to derivatives of the parent ligand. The phosphine products of the reaction are converted in situ to air-stable borane adducts for isolation, and versatile derivatisation reactions of these adducts are demonstrated.
- This article is part of the themed collection: Celebrating five years of ChemRxiv


 Please wait while we load your content...
Please wait while we load your content...