The convergent total synthesis and antibacterial profile of the natural product streptothricin F†
Abstract
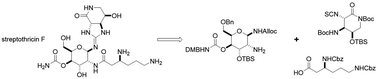
A convergent, diversity-enabling total synthesis of the natural product streptothricin F has been achieved. Herein, we describe the potent antimicrobial activity of streptothricin F and highlight the importance of a total synthesis that allows for the installation of practical divergent steps for medicinal chemistry exploits. Key features of our synthesis include a Burgess reagent-mediated 1,2-anti-diamine installation, diastereoselective azidation of a lactam enolate, and a mercury(II) chloride-mediated desulfurization-guanidination. The development of this chemistry enables the synthesis and structure–activity studies of streptothricin F analogs.


 Please wait while we load your content...
Please wait while we load your content...