Rongalite in PEG-400 as a general and reusable system for the synthesis of 2,5-disubstituted chalcogenophenes†
Abstract
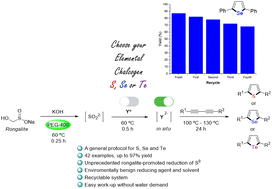
In this work, we report the use of rongalite in PEG-400 as a general, reusable, efficient, inexpensive and environmentally benign reductive system for elemental chalcogens and its use for the synthesis of 2,5-disubstituted chalcogenophenes (S, Se, Te) from 1,3-butadiynes. This non-conventional reductive system provided nucleophilic chalcogen species that could add regioselectively to the triple bond, with subsequent 5-endo-dig cyclization. Using this method, a wide range of symmetrical and non-symmetrical 1,3-butadiynes, bearing electron-donating or electron-withdrawing groups, were successfully employed for the synthesis of the desired chacogenophenes. This protocol benefits from many sustainable features; it is catalyst-free, safe to carry out, and uses cheap reagents and non-toxic chemical reaction media. Furthermore, the operationally simple reaction conditions and easy product separation allowed the reuse of PEG-400 in up to four successive reactions for the synthesis of 2,5-diphenylselenophene.


 Please wait while we load your content...
Please wait while we load your content...