Mechanistic differences between aryl iodide electrophiles and pronucleophiles in Pd-catalyzed coupling with cyclopropenes: a DFT study†
Abstract
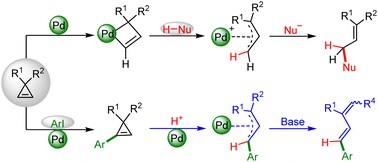
Computational studies were carried out to investigate the mechanisms of Pd-catalyzed ring-opening reactions of cyclopropenes with pronucleophiles (H-Nu) and aryl iodide electrophiles, respectively. The mechanistic path for the reaction of cyclopropenes with 2-methylmalononitrile mainly involves the ring-opening of cyclopropenes, isomerization, proton transfer, and nucleophilic attack. For the Pd-catalyzed arylation of cyclopropenes with aryl iodides, the mechanisms and chemo-selectivity are found to be dependent on the substituent attached to the C(sp3) atom of cyclopropenes. When a β-H atom is present in a group attached to the C(sp3) of cyclopropenes, the reaction could proceed successively through two catalytic cycles. The first cycle mainly includes the oxidative addition of aryl iodides, migration insertion of cyclopropenes, and base-assisted β-H elimination, leading to an aryl cyclopropene derivative. In the second cycle, after the substrate of aryl iodide is completely transformed, the Pd(0) catalyst would further activate the generated aryl cyclopropene to form a metallacyclobutene intermediate. Subsequently, the formed metallacyclobutene intermediate could sequentially undergo isomerization, proton transfer, and base-assisted β-H elimination to furnish the conjugated diene product. However, if such a β-H atom is absent, the second cycle cannot be performed. As a result, the final product is the aryl cyclopropene generated in the first cycle.


 Please wait while we load your content...
Please wait while we load your content...