Highly stereoselective synthesis of spirocyclopropylthiooxindoles and biological evaluation†
Abstract
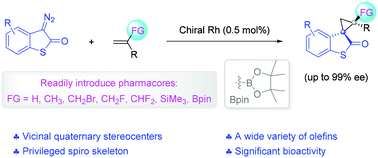
Stereochemically rich spirocycles are greatly sought-after structures for drug discovery. The development of efficient and flexible methods capable of constructing spirocycles derived from privileged scaffolds with structural diversity in a highly diastereo- and enantioselective manner is of current interest. Here, we present a novel highly stereoselective Rh-catalyzed olefin cyclopropanation of diazothiooxindoles for the facile access of optically active spirocyclopropylthiooxindoles in high to excellent enantiomeric excess. Notably, our protocol is compatible with a broad range of α-functionalized styrenes, allowing the merger of other pharmacophores such as fluoroalkyl, boron moiety, and silyl groups with spirocyclic thiooxindoles. Moreover, antiproliferative activity assessment shows that compounds 3e, 4a, and 4j displayed the most potent antiproliferation effect against MCF-7, HCT116, and SJSA-1 cells (IC50 = 1.09, 2.05, and 0.82 μM, respectively) without affecting normal cells.


 Please wait while we load your content...
Please wait while we load your content...