Electroreductive 4-pyridylation of unsaturated compounds using gaseous ammonia as a hydrogen source†
Abstract
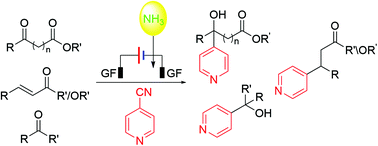
Electrochemical radical-cross-coupling with ammonia as a terminal reductant is reported in the reactions of α-Keto esters, β-keto esters, α,β-unsaturated esters, and α,β-unsaturated ketones with 4-CN pyridine. More than 50 corresponding pyridylation products were synthesized under constant current conditions using thiourea as an essential promoter to activate the intermediate derived from 4-CN pyridine.


 Please wait while we load your content...
Please wait while we load your content...