Antidiabetic profiling of veramycins, polyketides accessible by biosynthesis, chemical synthesis and precursor-directed modification†
Abstract
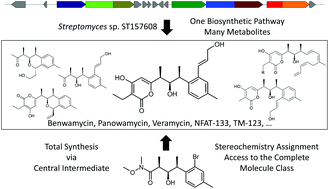
Seven new polyketides, termed veramycins, were isolated from a Streptomyces sp. from the Sanofi microbial strain collection along with their known congeners NFAT-133 and TM-123. Veramycin A, an α-pyrone congener of TM-123 and NFAT-133 showed an increased baseline deoxy-glucose uptake in the absence of insulin in a modified L6 rat skeletal muscle cell line (L6 GLUT4 AS160-like cells). In addition, both compounds slightly increased the sensitivity to insulin in this cell line. Total syntheses of NFAT-133, TM-123 and veramycin A were accomplished starting from a central building block, which bears the three contiguous stereogenic centers of this polyketide family. Our approach enables an efficient, selective and flexible access to all possible isomers of the stereotriad for further exploration of this series as a potential anti-diabetic lead structure as exemplified by the synthesis of an NFAT-133 epimer. Finally, the corresponding biosynthetic gene cluster (BGC) was identified by genome sequencing and gene inactivation. Based on feeding experiments, a biosynthetic pathway was proposed, which enabled access to new veramycin A analogs by precursor-directed biosynthesis.


 Please wait while we load your content...
Please wait while we load your content...