Synthesis of the C50 diastereomers of the C33–C51 fragment of stambomycin D†
Abstract
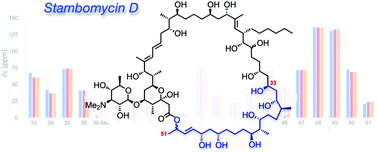
As products of genome mining, the stereochemical assignment of the macrolide antibiotics stambomycins A–D has been made on the basis of sequence analysis of the associated polyketide synthase, aside from two stereocentres at C28 and C50. Here we describe syntheses of the two C50 diastereomers of the C33–C51 region of the stambomycins, which support the PKS-based configurational assignment, and establish a strategy suitable for access to the extended stambomycin framework.


 Please wait while we load your content...
Please wait while we load your content...