Bromine and oxygen redox species mediated highly selective electro-epoxidation of styrene†
Abstract
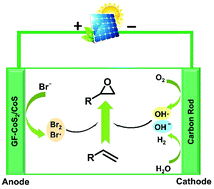
Olefin epoxidation is an essential transformation and arouses great interest among the scientific community for the key role of epoxide in the chemical industry. Traditional methods suffer from harmful oxidants and require harsh operating conditions and complex separation processes. Electro-epoxidation through bromine redox reactions is an eco-friendly strategy. However, the lack of efficient electrodes always limits the selectivity of epoxidation and results in massive energy consumption. Herein, we found that GF-CoS2/CoS heterostructures anchored on graphite felt (GF-CoS2/CoS) can efficiently facilitate the generation of bromine redox species on the anode. The synergistic cooperation between the bromine redox species and oxygen redox species (generated on a graphite rod cathode) can establish a benign electro-epoxidation system for olefin substrates in an ambient environment. Under the optimized reaction conditions, the epoxidation of styrene can achieve a yield of 97% at 30 mA cm−2. Importantly, the applied voltage (4–5 V) is roughly half of the Pt-based electrode system (7.8–9.3 V), hence, saving half of the energy. We demonstrated that our proposed strategy could also be applied to other olefins. Because of the simple, sustainable, and economic advantages of the epoxidation process, we believe this strategy is highly desirable for technical applications.


 Please wait while we load your content...
Please wait while we load your content...