Atropselective synthesis of N-aryl pyridones via dynamic kinetic resolution enabled by non-covalent interactions†
Abstract
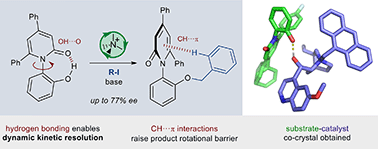
The dynamic kinetic resolution of C–N atropisomeric pyridones was achieved via asymmetric phase-transfer catalysis, exploiting a rotational barrier-lowering hydrogen bond in the starting materials. X-ray and NMR experiments revealed the presence of a barrier-raising ground state CH⋯π interaction in the product, supported by DFT calculations. A co-crystal of the quinidine-derived phase-transfer catalyst and substrate reveals key substrate–catalyst non-covalent interactions.

 Please wait while we load your content...
Please wait while we load your content...