Visible light-induced N-radical 5-exo/6-endo cyclization of alkenyl amides: facile access to isoindolinones/isoquinolinones†
Abstract
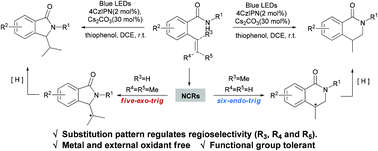
An efficient N-centered radical intramolecular cyclization reaction of alkenyl amides induced by visible light was described. In this process, an alkenyl amide underwent 5-exo/6-endo cyclization to selectively yield two critical alkaloid structures, namely isoindolinones and isoquinolinones.

 Please wait while we load your content...
Please wait while we load your content...