Synthesis of spirocyclic dihydropyrazoles from tosylhydrazones and electron-deficient alkenes†
Abstract
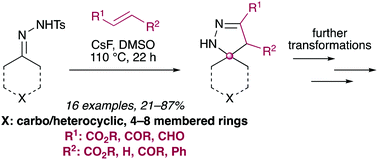
Spirocycles represent a diverse class of molecules which have received significant attention in the pharmaceutical industry due to their broad biological activities and inherent molecular three–dimensionality. Herein, we demonstrate a procedurally simple method for the preparation of a range of spirocyclic dihydropyrazoles. The protocol utilises bench stable cyclic tosylhydrazones, which are trivial to prepare from the parent cyclic ketone without need for purification, and commercially available electron deficient alkenes. The synthetic utility of the core scaffold is also demonstrated to highlight potential for applications in medicinal chemistry and drug development programmes.


 Please wait while we load your content...
Please wait while we load your content...