Direct catalytic benzene hydroxylation under mild reaction conditions by using a monocationic μ-nitrido-bridged iron phthalocyanine dimer with 16 peripheral methyl groups†
Abstract
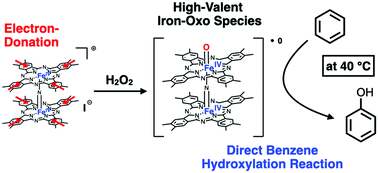
Direct catalytic hydroxylation of benzene under mild reaction conditions proceeded efficiently in the presence of a monocationic μ-nitrido-bridged iron phthalocyanine dimer with 16 peripheral methyl groups in an acetonitrile solution with excess H2O2. Mechanistic studies suggested that the reaction was catalyzed by a high-valent iron–oxo species generated in situ. Moreover, the peripheral methyl groups of the catalyst were presumed to have enhanced the production rate of the iron–oxo species.


 Please wait while we load your content...
Please wait while we load your content...