Ammonium iodide-catalyzed radical-mediated tandem cyclization of aromatic aldehydes, arylamines and 1,4-dioxane†
Abstract
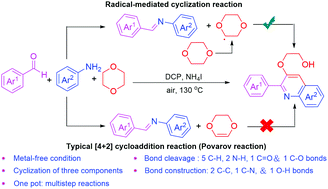
We have developed a novel approach for the construction of 2-((2-arylquinolin-4-yl)oxy)ethan-1-ol derivatives by an NH4I-catalyzed radical-mediated tandem cyclization of three components including aromatic aldehydes, arylamines and 1,4-dioxane. This strategy involves the breakage (C–H, N–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O bonds) and formation (C–C, C–N and O–H bonds) of multiple types of bonds. This transformation could involve a radical-mediated tandem cyclization reaction instead of the typical [4+2] cycloaddition reaction of imines with olefins. 1,4-Dioxane was used as a C4/O2 synthon to provide a 2-(vinyloxy)ethan-1-ol group.
O, and C–O bonds) and formation (C–C, C–N and O–H bonds) of multiple types of bonds. This transformation could involve a radical-mediated tandem cyclization reaction instead of the typical [4+2] cycloaddition reaction of imines with olefins. 1,4-Dioxane was used as a C4/O2 synthon to provide a 2-(vinyloxy)ethan-1-ol group.


 Please wait while we load your content...
Please wait while we load your content...